Patient Information
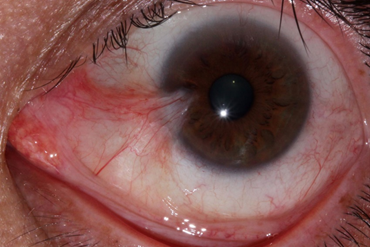 Pterygium is a commonly occurring ocular disease. Pterygium often occurs in regions with abundant sun exposure.
Pterygium is a commonly occurring ocular disease. Pterygium often occurs in regions with abundant sun exposure.
The pterygium patient population is estimated to be approximately 10 million to 15 million patients in the United States and approximately 10 million in Europe.
A pterygium is a benign fibrovascular growth on the cornea that can impair vision. It progressively grows and has cycles of irritation. It is associated with and thought to be caused by ultraviolet light exposure (e.g., sunlight).
Symptoms of pterygium includes hyperemia, irritation, foreign body sensation, and pain. It can cause visual impairment due to lesion obscuration on the visual axis.
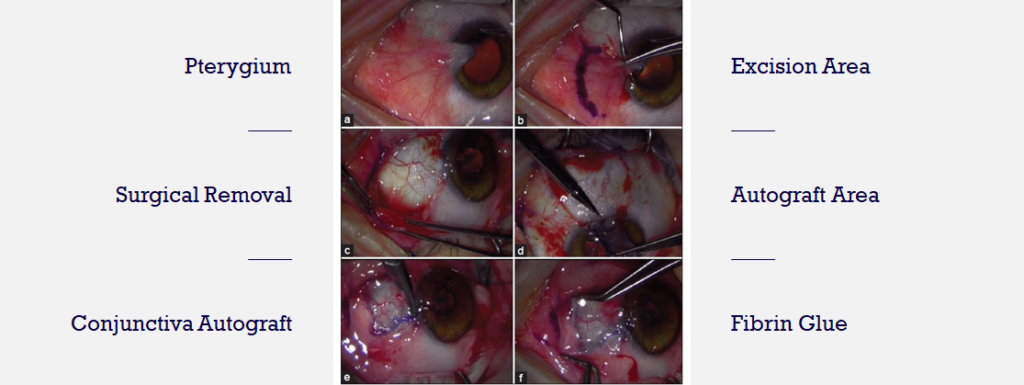
There are no FDA-approved drugs for pterygium. Physicians often prescribe off-label drugs including artificial tears, topical corticosteroids, and NSAIDs with limited efficacy or safety concern. Surgical removal is dependent upon visual axis involvement, symptom severity and cosmetic concerns.
The images above show the current standard of care to treat late stage pterygium patients – surgical excision. Unfortunately, post-surgical recurrence rate is high.
(Sources: 1. Medscape; 2. The National Renewable Energy Laboratory, Accessed November, 2018.; 3. Hovanesian, J. A. etc., J. Cataract Refract Surg., 2017, 43, 405-419; 4. Rezvan, F. etc., Surv Ophthalmol., 2018, 63, 719-735)