Pterygium
What is Pterygium?
Pterygium is a common ocular disease with high prevalence in regions with abundant sun exposure. The patient populations are estimated to be about 10 to 15 million in the United States and approximately 10 million in Europe.
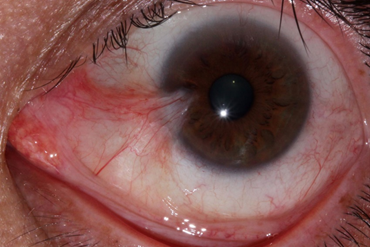
A pterygium lesion is an abnormal fibrovascular tissue growing onto the cornea that can impair vision. It often grows progressively and can impair vision, cause irritation and redness. The main cause of pterygium is ultraviolet light exposure (e.g., sunlight).
Sources:
- https://emedicine.medscape.com/article/1192527-overview#a6
- https://www.nrel.gov/gis/solar.html, Accessed November, 2018
- Hovanesian, J. A. etc., J. Cataract Refract Surg., 2017, 43, 405-419
- Rezvan, F. etc., Surv Ophthalmol., 2018, 63, 719-735
Current Treatment
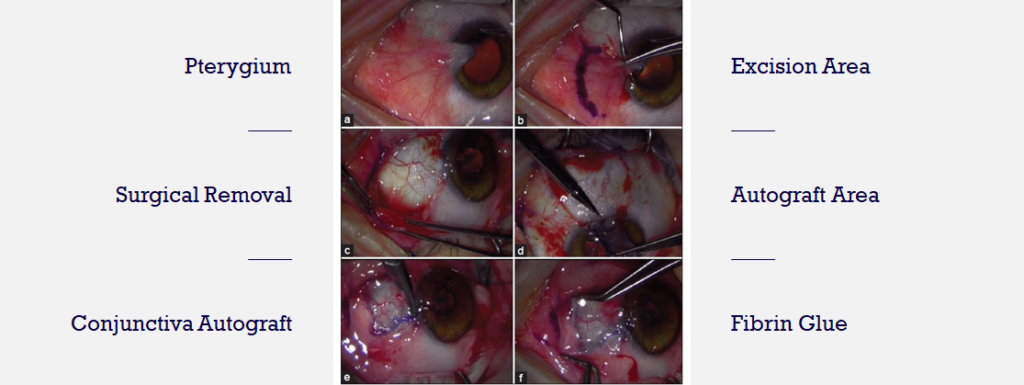
There are no FDA-approved drugs for pterygium. Physicians often prescribe off-label drugs including artificial tears, topical corticosteroids, or NSAIDs,These treatments are either ineffective or unsafe for long-term use. Surgical removal is dependent upon visual axis involvement, symptom severity and cosmetic concerns.
The images above show the current standard of care to treat late-stage pterygium – surgical excision. Unfortunately, post-surgical recurrence rate is still a problem.
Sources: 1. Medscape; 2. The National Renewable Energy Laboratory, Accessed November, 2018.; 3. Hovanesian, J.A.etc., J. Cataract Refract Surg., 2017, 43, 405-419; 4. Rezvan, F. etc., Surv Ophthalmol., 2018, 63, 719-735
Cloudbreak Solution
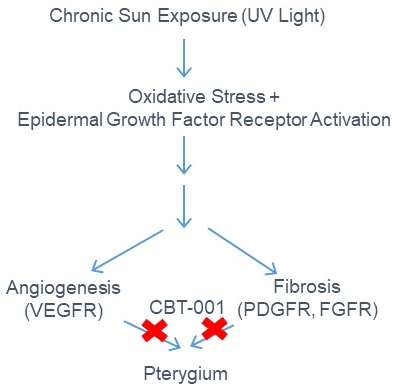
We formulated a potent multi-kinase inhibitor of VEGFRs, PDGFRs, FGFRs to target the well-established pterygia pathogenic mechanisms-angiogenesis and fibrosis. By targeting these pathways pharmacologically, the potent multi-kinase inhibitor will stop pterygium progression and eliminate the need for excision surgery. The image to the right shows the mechanism of action.
The pterygium program has completed a Phase 2 clinical trial with positive results. After a successful EOP2 meeting with the FDA in May 2019, Cloudbreak Pharma has begun Phase 3 clinical trials to develop CBT-001, a disease modifying, first-in-class drug candidate to treat pterygium. It will be the first drug therapy for this indication.