CBT-001
CBT-001 is currently in Phase 3 clinical trials to be evaluated for the treatment of pterygium. It will be the First Disease-Modifying, First-in-Class, Drug Therapy to Treat Pterygium.
Breakthrough Innovation
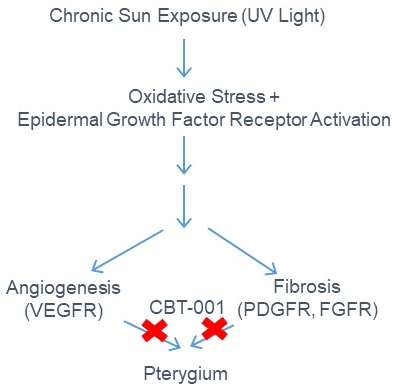
CBT-001 is a potent multi-kinase inhibitor of VEGFRs, PDGFRs, FGFRs, and can inhibit angiogenesis & fibrosis. CBT-001 is a unique topical formulation being developed via the 505b(2) regulatory pathway to treat pterygium that currently has no approved drug treatment. Surgical excision is the only option and the standard of care for pterygium patients. The angiogenic and fibrotic pathogenesis of pterygium is well established. By targeting these pathways pharmacologically, we will stop pterygium progression and eliminate the need for excision surgery.
Efficacy and Safety
In the Phase 2 clinical trial, CBT-001 substantially reduced pterygium vascularity and conjunctival hyperemia, in comparison to the control group, it also significantly regressed lesion length on cornea. In addition, CBT-001 is well tolerated in the eye and systemically. No patient withdrew due to adverse effects. There were no serious drug reactions or adverse effects. We had a successful EOP2 Meeting with FDA. The Phase 3 study plan was submitted for a special protocol assessment (SPA) and FDA agreed with our Phase 3 study plan.
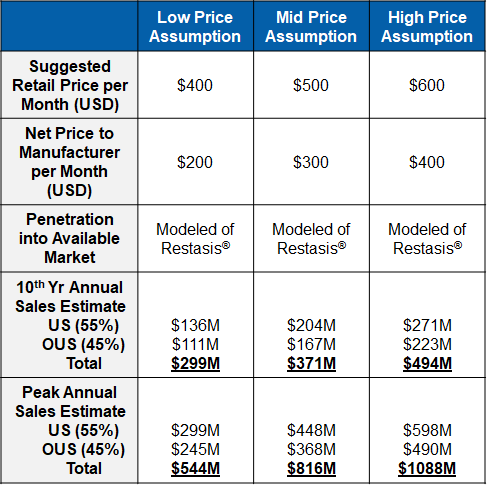
Market research supports a robust sales forecast for CBT-001 for the following reasons:
- CBT-001 addresses a largely unmet medical need
- It is a first-in-class and the first drug therapy to treat pterygium
- CBT-001 is a disease modifying therapy to regress pterygium size and reduce hyperemia
- It has quick onset for the treatment
- Its effect sustains for a long period
- It is a convenient self-dosing eye drop
- CBT-001 is an early treatment to avoid surgery or reduce recurrence rate post-surgery.