CBT-001
CBT-001 is currently in a Phase 3 clinical trial, being investigated as potentially the first disease-modifying topical treatment for pterygium.
Breakthrough Innovation
Our Core Product CBT-001 is a potential first-in-class drug therapy using multi-kinase inhibitor targeting platelet-derived growth factor receptors (“PDGFRs”), fibroblast growth factor receptors (“FGFRs”) and vascular endothelial growth factor receptors (“VEGFRs”), and is indicated for the prevention of pterygium progression and reduction of conjunctival
hyperaemia. It is expected to be able to address moderate to severe pterygium.
According to the F&S Report, there is currently no approved drug therapy for the treatment of pterygium globally, and the current existing treatment option for pterygium is surgical excision. CBT-001 is expected to be the first drug therapy globally for the treatment of pterygium, and to potentially reduce or postpone the need for surgical excision via early non-invasive
treatment to control pterygium progression, once approved.
Efficacy and Safety
Phase 2 Clinical Trial7
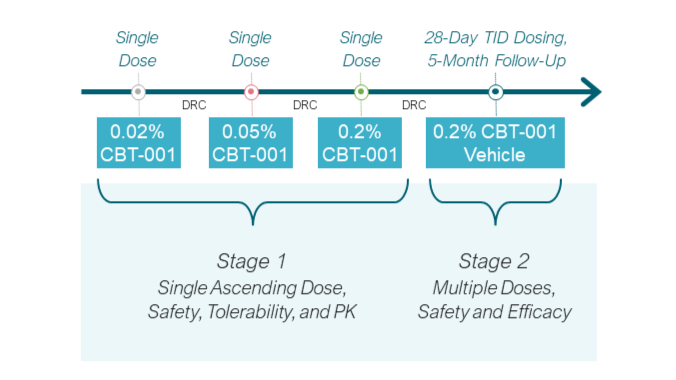
The Phase 2 clinical trial (NCT03049852) was divided into two stages. Stage 1 included a dose escalation of CBT-001 in 24 patients to determine the max tolerated dose. Results demonstrated ocular safety and tolerability with negligible systemic drug exposure. In Stage 2, 51 patients were treated with a 4-week cycle of TID dosing of CBT-001 (n=25) or vehicle (n=23) and were monitored through week 24. By week 4, a significantly decreased mean vascularity score was observed that remained through week 16. In addition, a significantly greater mean reduction in lesion length was observed at week 4 that remained through week 8.7
CBT-001 was well-tolerated, with the majority of commonly reported adverse events in the CBT-001 arm being ocular, mild in severity, and resolved after treatment. No patients discontinued CBT-001 treatment due to adverse events.7
Phase 3 Clinical Trial8
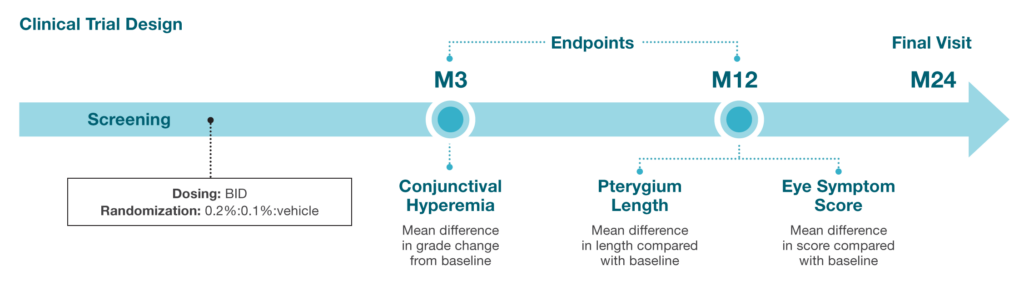
Phase 3 Study (NCT05456425) to evaluate the efficacy and safety of CBT-001 in patients with pterygium.8
Protocol Title: Multicenter, Double-Masked, Randomized, Vehicle-Controlled 12-Month Parallel Comparison of the Safety and Efficacy of 0.1% and 0.2% CBT-001 Versus Vehicle, Dosed Twice-Daily, in Patients With Pterygium
Study Objective: To evaluate the safety and efficacy of 0.1% and 0.2% CBT-001 emulsion dosed twice daily for 24 months compared to vehicle in reducing conjunctival hyperemia and preventing pterygium progression in eyes with pterygia
Target Enrollment: 600 patients
Key Inclusion Criteria:
- Male or female ≥12 years of age
- Pterygium lesion length ≥1.2 mm over the cornea
- Conjunctival hyperemia of Grade ≥3
Key Exclusion Criteria:
- Pterygium removal surgery within the last 6 months
- Anticipated pterygium surgery within a year of study enrollment
- Clinically significant corneal abnormalities other than pterygium
This phase 3 clinical trial is currently enrolling. More information can be found on ClinicalTrials.gov for NCT05456425 or
you can email CBT-CS301Study@cloudbreakpharma.com for additional information.
BID, twice daily; DRC, data review committee; FGFR, fibroblast growth factor receptor; M, month; PDGFR, platelet-derived growth factor receptor; PK, pharmacokinetics; TID, three times daily; VEGFR, vascular endothelial growth factor receptor.
1. Shahraki T, et al. Ther Adv Ophthalmol. 2021;13:25158414211020152. 2. Chu WK, et al. Eye (Lond). 2020;34(6):1047-1050. 3. Wanzeler ACV, et al. Clin Ophthalmol. 2018;12:833-837. 4. Palewski M, et al. Int J Environ Res Public Health. 2022;19:11357. 5. Hilberg F, et al. Cancer Res. 2008;68:4774–4782. 6. Yang R, et al. Invest Ophthalmol Vis Sci .2019;60(9):2087. 7. Whitcup SM, et al. Ophthalmol Sci. 2024. Publication in press. https://doi.org/10.1016/j.xops.2024.100502. 8. ClinicalTrials.gov. A clinical trial on safety and efficacy of CBT-001 in patients with pterygium. Updated March 15, 2024. Accessed March 15, 2024. https://clinicaltrials.gov/study/NCT05456425.