Discover our Products in U.S. Clinical Trial
Clinical trials are research studies that are designed to determine if a medicine is safe and effective for patients.
Phase 3 Study to Evaluate the Efficacy and Safety of CBT-001 in Patients with Pterygium
Condition: Conjunctival Hyperemia Reduction and Pterygium Lesion Regression
Phase: Phase 3
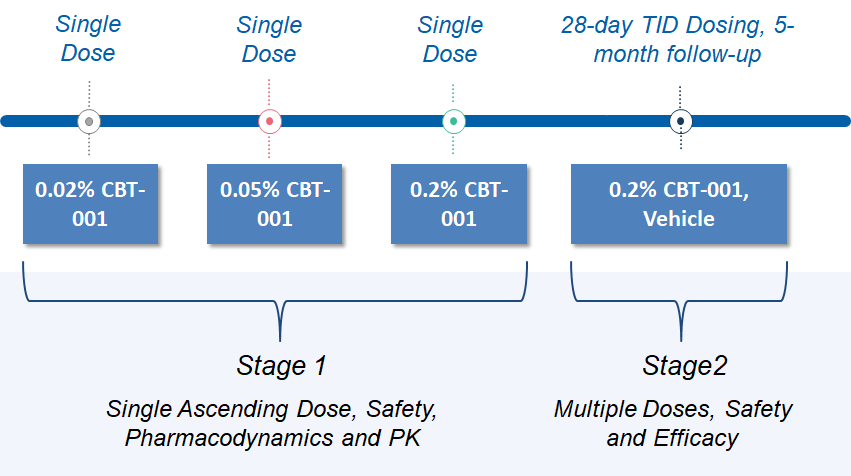
A previous Phase 2 study had completed. In that study, a CBT-001 ophthalmic solution was evaluated in two stages. Stage 1 was a single ascending dose study to evaluate the safety, efficacy, and pharmacokinetics of 0.02%, 0.05% and 0.2% CBT-001. )Stage 2 assessed the efficacy and safety of 0.2% CBT-001, administered three time daily (TID) for 4 weeks with a 5 months follow up. The primary endpoint was reduction of pterygium vascularity and the key secondary endpoint was inhibition of lesion growth. Both endpoints showed positive results. The results of the study can be found on ClinicalTrials.gov. After the completion of the trial, we had a successful EOP2 Meeting with FDA.
The Phase 3 study was submitted for SPA and FDA accepted the protocol with amendment.
Phase 3 Study Title: Multicenter, Double-Masked, Randomized, Vehicle-Controlled 12-Month (with a 12-month, Double-Masked Extension) Parallel Comparison of the Safety and Efficacy of 0.1% and 0.2% CBT-001, Dosed Twice-Daily, in Patients with Pterygium.
The objective is to evaluate the safety and efficacy of 0.1% and 0.2% CBT-001 emulsion dosed twice daily for 24 months compared to vehicle in reducing conjunctival hyperemia and preventing pterygium progression in eyes with pterygia.
Primary Efficacy Endpoints are focused on the reduction of conjunctival hyperemia caused by pterygium and inhibition of lesion length progression.
More detailed information about this Phase 3 study can be found on https://clinicaltrials.gov/study/NCT05456425
A second Phase 3 study is planned for early 2026. Please check ClinicalTrials.gov for more details.
Study Design
Please visit clinical trials.gov website to learn more about CBT-001 trial, and our Patient Information page to learn more about pterygium.
Primary Endpoint was Met with Highly Statistical Significance
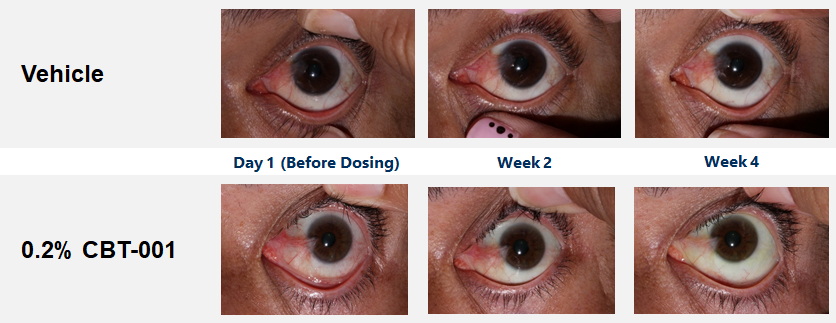
CBT-001 demonstrates good ocular and systemic safety, quick onset, and highly effective during and post-dosing.
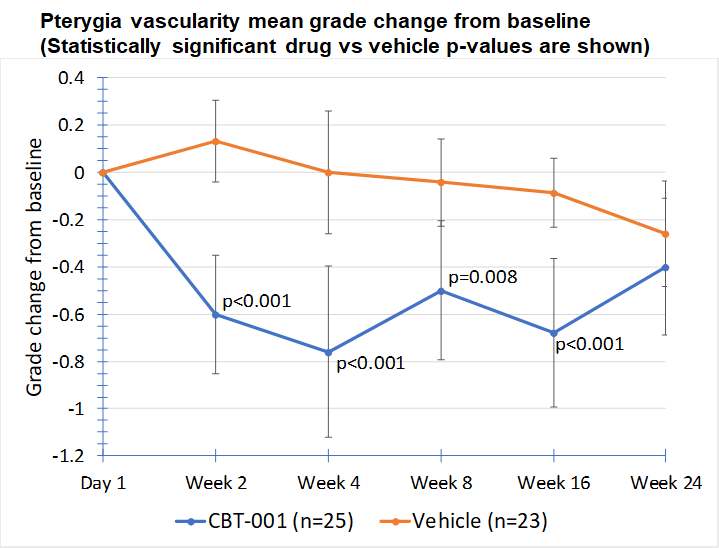
Baseline demographic characteristics were similar between patients receiving CBT-001 (n=25) and vehicle (n=23). After four (4) weeks of dosing, mean vascularity scores were significantly decreased in patients receiving CBT-001 (-0.8) compared to vehicle (0.0) (p<0.001). Vascularity remained significantly decreased at weeks 8 (p=0.008) and 16 (p<0.001), but not at week 24. The CBT-001 group showed significantly greater mean reductions in lesion length at week 2 (p=0.005), week 4 (p=0.007) and week 8 (p=0.0145).
EOP2 Meeting with FDA
Proceed with Phase III Trials after a Successful EOP2 Meeting with FDA Regarding CBT-001 as a Treatment for Pterygium
We had a successful EOP2 meeting with the FDA. The FDA agreed with proceeding to Phase III trials, agreed on Phase III study design and efficacy endpoints, and agreed on CMC, non-clinical, and clinical plan.