我们的产品在美国临床试验中
临床试验是旨在确定药物对患者是否安全有效的研究。
Phase 3 评估 CBT-001 在翼状胬肉患者中的疗效和安全性的研究
状况: 血管化的睑裂斑
阶段:第 3 阶段
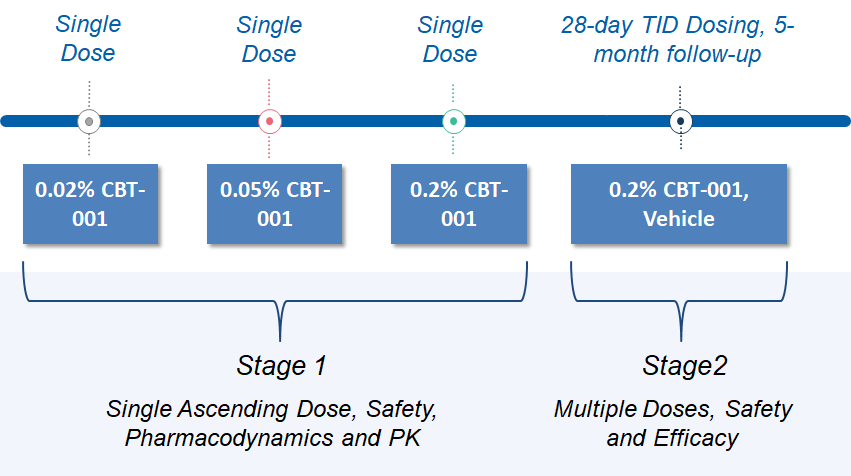
之前的 2 期研究已经完成。在该研究中,分两个阶段评估了CBT-001滴眼液。第 1 阶段是一项单次递增剂量研究,以评估 0.02%、0.05% 和 0.2% CBT-001 的安全性、有效性和药代动力学。)第 2 阶段评估了 0.2% CBT-001 的疗效和安全性,每天 3 次 (TID),持续 4 周,随访 5 个月。主要终点是翼状胬肉血管减少,关键次要终点是抑制病变生长。两个终点均显示阳性结果。研究结果可以在 ClinicalTrials.gov 上找到。试验完成后,我们与FDA成功举行了EOP2会议。
3期研究已提交SPA,FDA接受了修改后的方案。
3 期研究标题:多中心、双盲、随机、载体对照 12 个月(延长 12 个月,双盲)平行比较 0.1% 和 0.2% CBT-001 的安全性和有效性,每日两次,在翼状胬肉患者中。
目的是评估 0.1% 和 0.2% CBT-001 乳液与载体相比,每天两次给药 24 个月在减少翼状胬肉眼中的结膜充血和预防翼状胬肉进展方面的安全性和有效性。
主要疗效终点集中在减少由翼状胬肉引起的结膜充血和抑制病变长度增加进展。
有关此 3 期研究的更多详细信息,请访问 https://clinicaltrials.gov/study/NCT05456425
A second Phase 3 study is planned for early 2026. Please check ClinicalTrials.gov for more details.
Study Design
Please visit clinical trials.gov website to learn more about CBT-001 trial, and our Patient Information page to learn more about pterygium.
Primary Endpoint was Met with Highly Statistical Significance
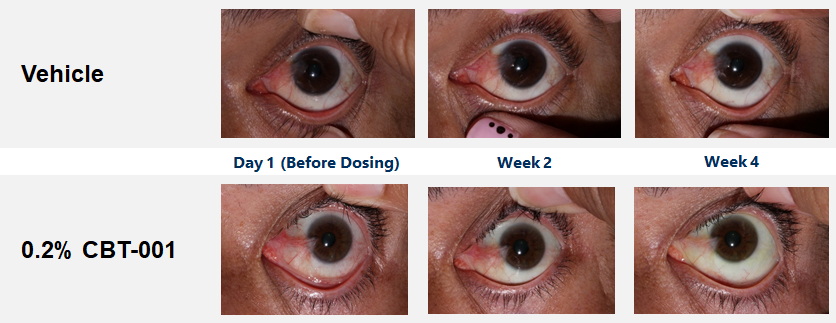
CBT-001 demonstrates good ocular and systemic safety, quick onset, and highly effective during and post-dosing.
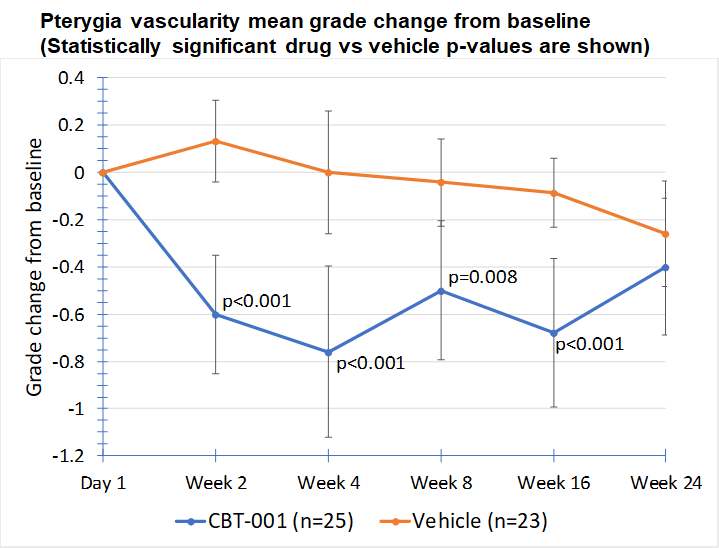
Baseline demographic characteristics were similar between patients receiving CBT-001 (n=25) and vehicle (n=23). After four (4) weeks of dosing, mean vascularity scores were significantly decreased in patients receiving CBT-001 (-0.8) compared to vehicle (0.0) (p<0.001). Vascularity remained significantly decreased at weeks 8 (p=0.008) and 16 (p<0.001), but not at week 24. The CBT-001 group showed significantly greater mean reductions in lesion length at week 2 (p=0.005), week 4 (p=0.007) and week 8 (p=0.0145).
EOP2 Meeting with FDA
Proceed with Phase III Trials after a Successful EOP2 Meeting with FDA Regarding CBT-001 as a Treatment for Pterygium
We had a successful EOP2 meeting with the FDA. The FDA agreed with proceeding to Phase III trials, agreed on Phase III study design and efficacy endpoints, and agreed on CMC, non-clinical, and clinical plan.